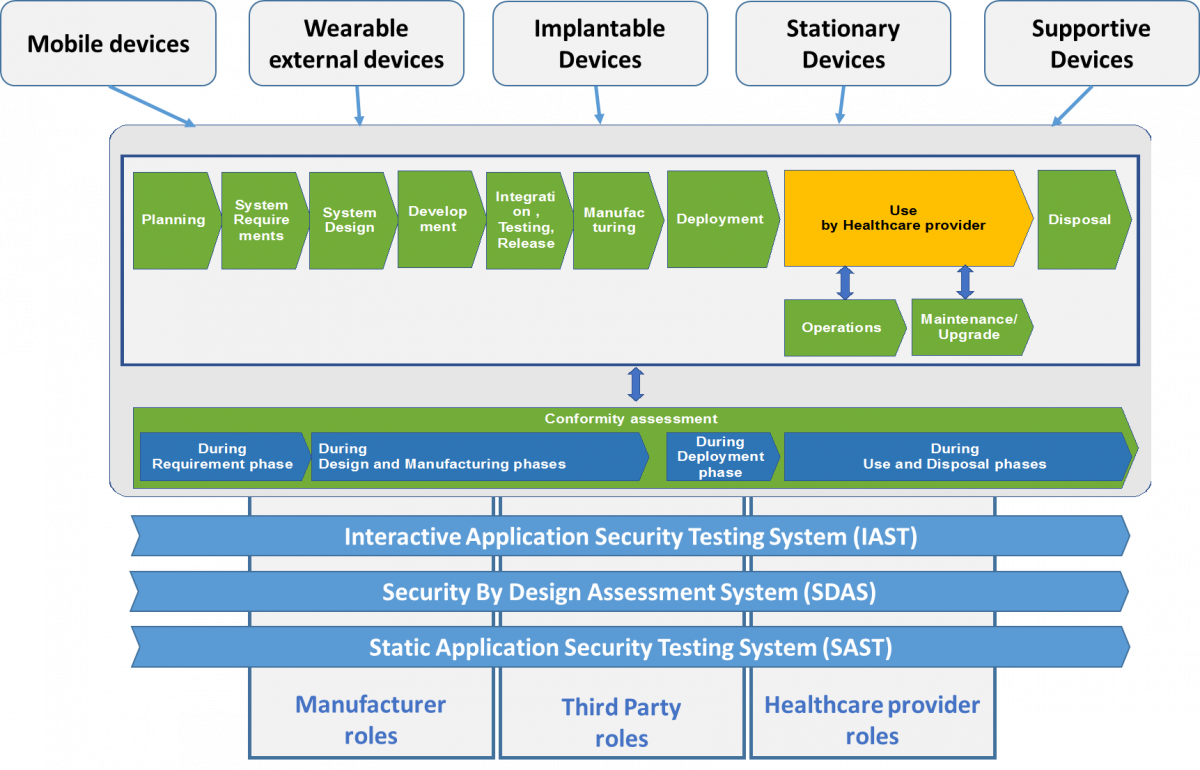
4.b Security by design - Medical Devices (SUPPLIER)
Definition
Approach to software and hardware development that seeks to make systems as free of vulnerabilities and impervious to attack as possible through such measures as continuous testing, authentication safeguards and adherence to best programming practices.
Objectives of the topic session
- Understanding the needs to cover cyber-security aspects during medical devices lifecycle
- Identification of the critical phases that are required of the medical devices lifecycle with regard to cyber-security
- Understanding the key actors in HCO and medical devices suppliers and how it might be grouped, e.g. according to level of responsibility, role, health sector context, etc.
- Information on previous knowledge/experience and any common/local policy or standards that will apply to the medical devices lifecycle